January 31, 2025—
In response to growing concerns about the risks of Ethylene Oxide (EtO) exposure, the EPA has issued an Interim Decision (ID) under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) for the pesticidal uses of EtO. This decision highlights the need for new risk mitigation measures, identifies necessary data for further reviews, and outlines actions required to improve safety. Montrose Environmental Group, alongside its innovative Sensible Environmental Data Platform (EDP), is uniquely positioned to help facilities navigate these changes and ensure compliance.
What is the Interim Decision?
The EPA’s Interim Decision (ID) on EtO specifically addresses its use in commercial sterilization, a process critical for the medical device and pharmaceutical industries. The ID aims to reduce the risks associated with EtO exposure by laying out new safety measures and revising existing ones. Key aspects of the ID include:
-
-
-
- •Risk Mitigation Measures: These measures will apply to several industries, including healthcare and commercial sterilization, as well as certain research and development facilities using EtO.
-
- •Exposure Assessment: The ID evaluates the exposure risks for various groups, including occupational, occupational bystanders, non-residential bystanders, and residential bystanders. It emphasizes the need to protect workers and surrounding communities from potential harm.
-
Key Changes in the Interim Decision
The EPA’s review has led to the following key updates and requirements:
-
-
-
- 1. Ban on Certain Uses: The ID mandates the cessation of EtO use for certain materials, including museum artifacts, cosmetics, and beekeeping equipment.
-
- 2. Concentration Limits for Medical Devices: The proposed concentration limit for EtO in medical device sterilization has been updated. A concentration of 600 mg/L is now allowed for new cycles (with a deadline of January 1, 2035), with exceptions for existing cycles that have FDA approval above this level.
-
- 3. Stronger Air Quality Monitoring: Continuous air monitoring will now require a threshold of 0.1 ppm (100 ppb) for stationary indoor air quality monitoring. This includes the visibility of monitoring results to workers to ensure safety. Monitoring will need to be implemented within one year.
-
- 4. 2-Minute Sample Duration: Montrose interprets this requirement as referring to the sample duration rather than the sampling frequency. We are actively seeking clarification from FIFRA and will update customers as soon as we receive confirmation regarding the 2-minute interval language.
-
- 5. Key Compliance Deadlines for FIFRA Regulations:
-
-
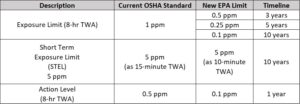
- a. January 1, 2026: Facilities must implement continuous monitoring systems to track personnel exposure and display real-time EtO concentrations (reference: Pg. 63, Table 3 & 4). The 8-hour time-weighted average (TWA) limit is set at <1 ppm.
- b. January 1, 2028: TWA compliance lowers to <0.5 ppm over an 8-hour period.
- c. January 1, 2030: TWA compliance lowers further to <0.25 ppm.
- d. January 1, 2035: The final TWA limit is <0.1 ppm.
-
- 6. Personal Protective Equipment (PPE) Updates: PPE protocols for workers exposed to EtO have been revised. Respirators are required when exposure exceeds the updated occupational limits, with specific requirements based on time-weighted averages (1 ppm for an 8-hour shift and 5 ppm for short-term exposure).
-
- 7. Enhanced Worker Protections: Increased monitoring, medical surveillance, and training are now required for workers exposed above the updated action levels. This includes annual worker exposure data collection and adherence to revised safety protocols.
-
-
- Summary of Worker Exposure Limit, Short Term Exposure Limit, and Action Level for EtO Commercial Sterilization Facilities
-
How Montrose and Sensible EDP Can Help
Montrose Environmental Group provides an integrated solution to help sterilization facilities meet the challenges of new EtO regulations. Combining Montrose’s regulatory expertise with Sensible EDP’s advanced technology, we deliver comprehensive support tailored to your facility’s compliance needs.
Comprehensive Solutions for EtO Compliance
•Facility Assessments: Evaluate current EtO usage and emissions against updated EPA thresholds.
•Exposure Mitigation Strategies: Develop tailored solutions to minimize occupational and bystander risks, such as separating HVAC systems and implementing PPE protocols.
•Regulatory Expertise: Stay ahead of FIFRA and NESHAP compliance deadlines with expert support in data submissions and registration reviews.
•Health and Safety Stewardship: Reduce risks while protecting the health of workers and surrounding communities through innovative and reliable solutions.
Real-Time Monitoring and Data Management with Sensible EDP
•Real-Time Monitoring: Track indoor air quality and verify compliance with revised 0.1 ppm monitoring thresholds.
•Custom Alerts: Get instant notifications for threshold exceedances, enabling immediate corrective action.
•Data Aggregation and Reporting: Streamline compliance reporting through automated data collection from stationary monitors, wearable devices, and other sources.
•24/7 Backup and Redundancy: Ensure consistent monitoring and data integrity with reliable system support.
Together, Montrose and Sensible EDP deliver an integrated approach to EtO compliance. Montrose’s team of experts brings deep regulatory knowledge and industry experience, while Sensible EDP’s cutting-edge technology empowers facilities to meet rigorous standards efficiently and effectively. With this dual support, sterilization facilities can achieve compliance, safeguard health and safety, and contribute to environmental stewardship.
Take the next step in compliance today. Contact us to learn how we can help your facility seamlessly navigate these changes.